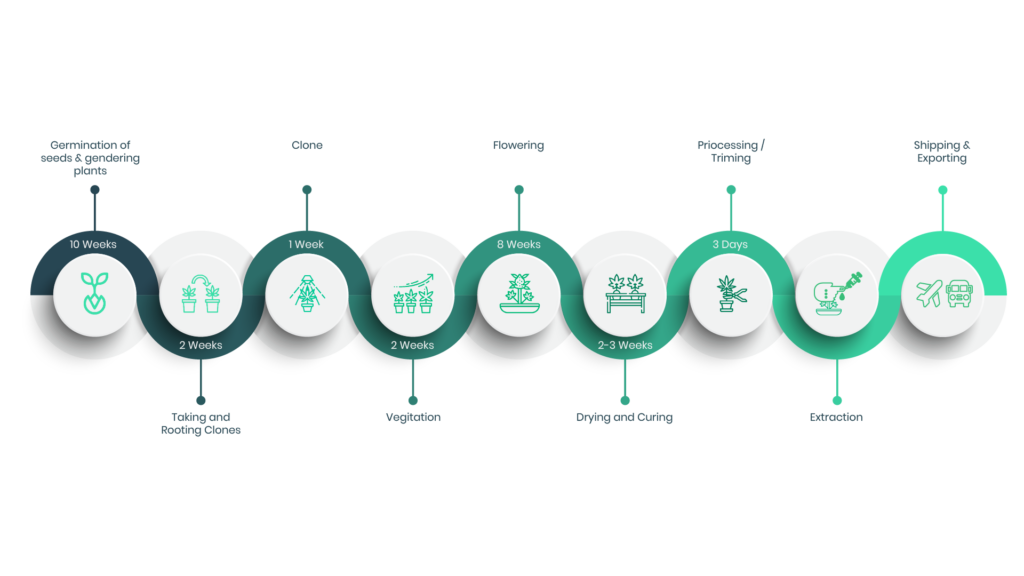
Production
At CINV, we meticulously craft premium medical cannabis cultivars, tailored for therapeutic benefits. Our advanced South African facilities ensure we have control and oversight on every step, from seed to sale.
Cultivation Expertise
With over six decades of combined cultivation experience, our team understands how to craft the perfect formula for exceptional cannabis flower. From ideal structure to cannabinoid levels, terpene profiles, and flavour, we breed specialised varieties to unlock rare compounds for tailored cannabinoid profiles for the complete entourage effect.

Production Process

Cutting-Edge Facilities
Our subsidiary Ember Pharms conducts breeding, propagation, preservation, and R&D at a licensed greenhouse facility. The site currently boasts 17,000 sqm of land, advanced processing facilities, and licences for cultivation. With 1800m2 of greenhouse and a 1370m2 indoor facility, we anticipate 2200-3000kg of annual capacity. We plan to build an EU GMP-certified indoor cultivation site to increase capacity up to 9,360 kg annually by 2026.
Strategic Partnerships
We partner with additional out-grow facilities to escalate our production capacity, onboarding them into our strategic framework. To discuss partnership opportunities, please get in touch.


End-to-End Quality Control
Our team directly oversees every harvest and ensures rigorous testing and compliance are undertaken through our third-party partners. Our seed-to-heal framework includes EU-GMP-certified processing, export, and distribution, via either a schedule 2 narcotics-certified dispensary or registered CQC clinics.